AMTicide® VAF
Background
Consumer demand and ever-changing regulations has driven the market from synthetic material to focus on natural solutions. Active Micro Technologies prides itself in developing and supplying effective, natural products that provide skin and hair conditioning benefits, along with providing natural antimicrobial activity. AMTicide® VAF is developed by co- fermenting Bacillus subtilis with Saccharomyces boulardii in a defined growth media to deliver a non-irritating, effective, multifunctional product. This highly marketable product can provide antioxidant benefits and is capable of preventing the growth of fungus in packaging headspace, making it the perfect addition to any formulation.
Science
Active Micro Technologies has identified a gap in the market for a naturally derived volatile antifungal to prevent microbial growth associated with packaging. Packaging is a critical component for the successful preservation of cosmetic and personal care products. For a variety of packaging options, including jars, ensuring that the packaging headspace remains free of contamination is essential to prevent contamination of the entire formulation. Packaging processes are able to create a favorable growth environment for yeast and mold.
For example, leaving space between the contents of the packaging and the lid of the packaging allows suficient room for microbial growth after a hot poured formulation has cooled. In addition, each time the packaging container is opened and the product is used, the headspace in the packaging increases, exposing more surface area of the formulation to the air. Phenoxyethanol, a synthetic volatile antimicrobial, has been commonly utilized to prevent headspace microbial growth. However, the potential for sensitization and sensory irritation of the skin associated with the use of phenoxyethanol, as well as stricter worldwide regulations on the material, pushes formulators to explore alternative options. Active Micro Technologies has successfully provided a natural volatile antifungal solution with the development of AMTicide® VAF.
AMTicide® VAF is a product of the co-fermentation of Bacillus subtilis and Saccharomyces boulardii in a defined growth medium. Bacillus spp. are well known rhizosphere residents of many crops, including tomato, corn, and soybeans, and produce nonvolatile and volatile secondary metabolites that exhibit antifungal activity as a mechanism of biocontrol to promote plant growth. The volatile organic metabolites produced by Bacillus subtilis have been known to naturally reduce and prevent plant diseases caused by fungi.
Saccharomyces boulardii is a probiotic strain of yeast, first isolated from lychee and mangosteen fruit in 1934 by a French scientist by the name of Henri Boulard. Saccharomyces boulardii has sparked interest around the world, specifically due to its wide variety of interactions with other microbes and ability to disable gastrointestinal disorder and symptoms of gastrointestinal distress. Current research in the food industry has examined the ability of Saccharomyces spp. and lactic acid bacteria, such as Bacillus spp., to enhance the production of volatile compounds when co-inoculated.
Active Micro Technologies has included the inoculation of Saccharomyces boulardii in the fermentation process to enhance the bioactivity of the Bacillus subtilis volatile metabolites. Using bio-fermentation and various filtration techniques, the volatile organic metabolites are isolated and extracted from the bacteria cell to deliver high potency volatile antifungal activity. Active Micro Technologies has been able to successfully produce a naturally derived, high potency volatile material that delivers moisturizing and antifungal activity for effective headspace protection!
AMTicide® VAF was developed to be used in conjunction with one of our broad-spectrum antimicrobials, however it can be used alongside any preservative package for extra protection against yeast and mold associated with packaging.
Benefits
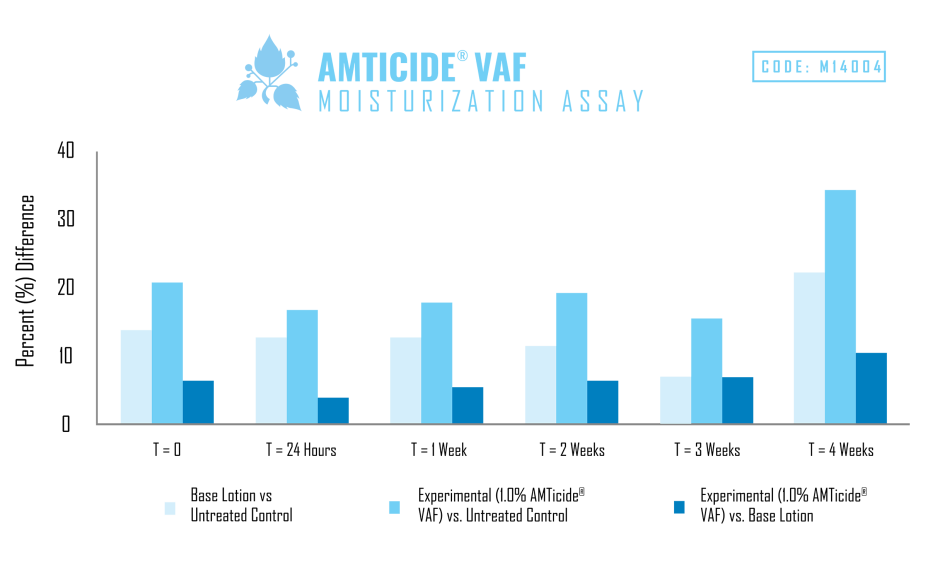
A skin moisturization study was performed using an untreated control, generic cream base, and an experimental with the same cream base containing 1.0% AMTicide® VAF.
As demonstrated by the results of this study, the addition of 1.0% AMTicide® VAF improved moisture levels by 16.50% after 24 hours and by 34.0% after four weeks when compared to the untreated control. When compared to the base cream AMTicide® VAF improved moisturization by 3.96% and after 24 hours and by 9.88% after four weeks. Results indicate that AMTicide® VAF is capable of increasing moisturization when compared to both the untreated control as well as the base lotion.
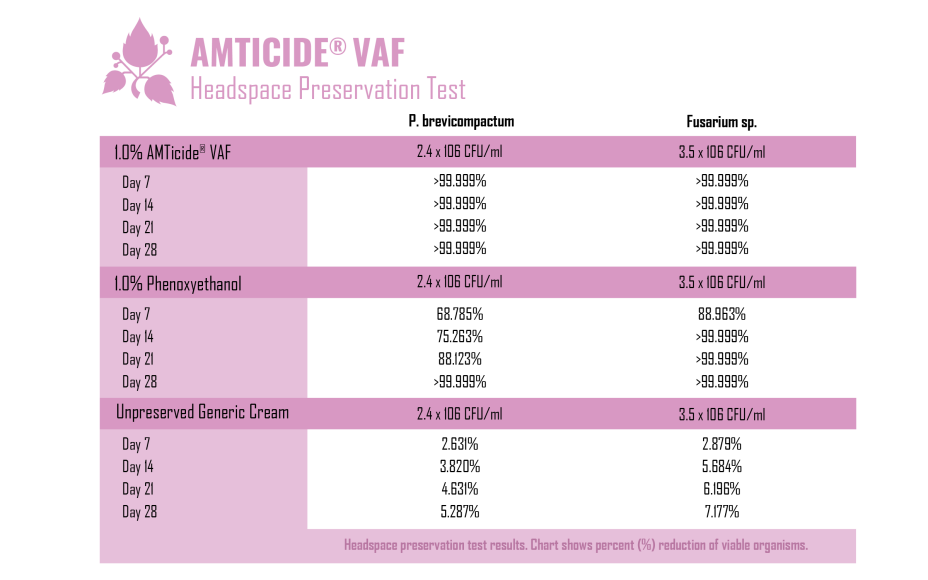
A Headspace Preservation Test was then conducted over a period of 28 days to evaluate the preservation adequacy of 1.0% AMTicide® VAF compared to phenoxyethanol (positive control) and an unpreserved generic cream base (negative control). Penicillium brevicompactum and Fusarium sp. were isolated from the environment via passive sedimentation, to observe a ‘real-life’ example of contamination from manufacturing areas, storage conditions, or consumer use.
The base cream formula used to perform the test was poured into individual cosmetic containers at 45oC in order to create the best environment for mold to grow under the test conditions. Each plastic jar was filled to approximately 85% of its capacity (~25ml) leaving 25% capacity of each jar empty as headspace. 1.0% AMTicide® VAF and 1.0% phenoxyethanol were subsequently added separately. Each cap of the cosmetic containers was inoculated separately with Penicillium brevicompactum and Fusarium sp. Each cap was tested at 7, 14, 21 and 28 days after the initial inoculation.